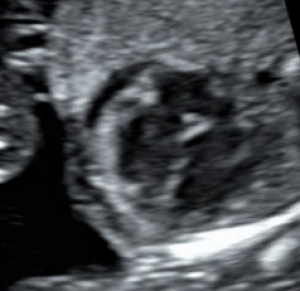
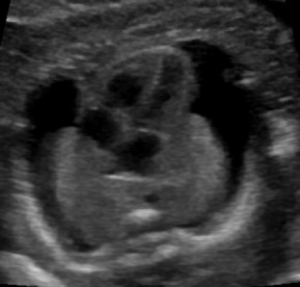
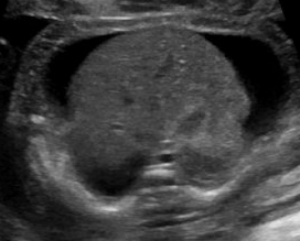
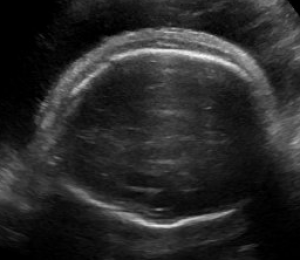
Hydrops fetalis is diagnosed during pregnancy when abnormal fluid collections are seen in the fetus, such as around the heart or lungs, in the abdomen, or in the skin and soft tissues. It carries significant risks of stillbirth, preterm delivery, maternal illness, and serious illness or death for the newborn.1-6 However, these risks and the approach to management vary widely by the underlying cause.
There are two types of hydrops fetalis: one results from blood type incompatibility between the woman and fetus (referred to as alloimmunization). When Rh(D) immune globulin is administered appropriately though, only 10% of contemporary hydrops fetalis cases result from this cause. The other 90% result from non-immune causes (referred to as non-immune hydrops fetalis, NIHF) that include genetic disorders, birth defects, viral infections, and unknown reasons.1 The remainder of this review is focused on the causes of and management for NIHF in singleton pregnancies. For a discussion of hydrops in the setting of twin pregnancies, please refer to the separate page on twin-twin transfusion syndrome.
Prevalence
NIHF affects between 1:1700 to 1:3000 pregnancies overall.1 This prevalence may be higher among populations with a greater number of individuals who are carriers of genetic disorders associated with NIHF.
Diagnosis and Evaluation
Abnormal fluid collections in the fetus leading to a diagnosis of NIHF can be seen on routine prenatal ultrasound. They may include fluid around the heart (pericardial fluid), around the lungs (pleural effusion), in the abdomen (ascites), or in the skin and soft tissues (skin edema). An increased amount of amniotic fluid (polyhydramnios) and an enlarged placenta (placentomegaly) may also be seen, although these findings are not part of the formal diagnostic criteria for NIHF.
When NIHF is detected, it is important to perform a thorough evaluation to find the underlying cause. A detailed obstetric and family history are essential for assessing risk factors for NIHF. The evaluation also includes obstetric ultrasound, fetal heart ultrasound (echocardiogram), maternal blood tests including mean corpuscular volume, testing for viral infections, genetic testing with chromosomal microarray or karyotype from amniocentesis, and more specific genetic testing if indicated.1
Fetal MRI is also becoming increasingly useful to further understand certain types of birth defects. Even in cases where a birth defect is identified, further evaluation with genetic testing is essential to better understand outcomes for that fetus as well as risk of NIHF recurring in a future pregnancy. After delivery, a detailed examination of the placenta can provide useful information about a cause for the NIHF. For cases that do not survive pregnancy, autopsy can also provide important information if families choose to pursue this option.
Despite thorough evaluations with these standard tests, a clear cause is not identified in approximately half of all NIHF cases.2 Consultation with a high risk obstetrician or geneticist at a center familiar with NIHF is an important part of the evaluation process, in order to understand further options for testing (such as NIHF gene panels or trio exome sequencing),7 management options, and what to expect both during and after pregnancy. Prenatal consultation with specialists important for the care of the neonate after birth is also helpful for some families, such as in situations where the fetus has a cardiac defect and will need surgery after birth.
Management Options and Outcomes
Developing a management plan for NIHF depends on the results of a thorough evaluation. For some genetic disorders causing NIHF, interventions are available during pregnancy and after birth to improve outcomes. Examples include alpha thalassemia for which intrauterine transfusions improve outcomes during pregnancy,8 and enzyme replacement therapies that can be started soon after birth for inborn errors of metabolism. For many causes of NIHF, however, there is not yet a specific treatment either before or after birth. For cases in which a cause is not found, careful management with the surveillance plan below is still indicated.
Consultation should be obtained from a high risk obstetrician regarding management of pregnancies affected by NIHF. Centers with expertise in fetal treatment can provide recommendations about fetal interventions if appropriate. For example, in cases of a very large fluid collection around the fetal lungs (pleural effusion), consideration may be given to placement of a shunt in utero to drain the fluid collection.
Pregnant women should be monitored very closely throughout a pregnancy with NIHF, as there is risk of developing a condition called maternal mirror syndrome (a form of preeclampsia). With this syndrome, the mother “mirrors” the fetus by developing swelling in some cases, and/or high blood pressure, abnormal liver or kidney tests, protein in the urine, headache, and/or other abnormalities. Blood pressure should be checked frequently, along with lab work. If maternal mirror syndrome develops, delivery becomes indicated for the safety of the mother and fetus.
Approximately two thirds of pregnancies with NIHF are delivered preterm (before 37 weeks gestation).1 This may be because of spontaneous labor, or because complications such as maternal mirror syndrome or significantly worsened NIHF develop and necessitate an early delivery. Corticosteroids are often recommended to decrease the risk of neonatal respiratory distress and other complications if concern arises for a preterm delivery. However, in the absence of spontaneous preterm labor, maternal illness, significantly worsened NIHF, or other complicating factors, current recommendations are to aim for a term delivery to minimize the additional risks of prematurity.
Antenatal testing (also called non-stress testing) is usually started in the third trimester, to monitor the well-being of the fetus in utero. Serial ultrasounds to assess fetal growth, the abnormal fluid collections, and other parameters are also recommended. Delivery should be planned at a tertiary care center with access to high risk obstetricians, neonatologists, and other specialists important for care of the neonate after birth.
Prognosis for neonates born with NIHF varies widely by the underlying cause, as well as with gestational age at delivery, extent of resuscitation at delivery, and need for transport to a higher level center.1 Overall survival is generally less than 50%.1 However, it is higher for treatable cases such an abnormal fetal heart rate (fetal arrhythmia) or infection with parvovirus, and lower for cases of a severe birth defect. When the prognosis is felt to be extremely poor with very little to no chance of survival, providers may discuss the option of comfort care (meaning less interventions) with families.
Recurrence Risk
Counseling about recurrence risk for NIHF in a future pregnancy is largely dependent on understanding why it happened in the first place. For cases resulting from an autosomal recessive genetic disorder (where both parents are carriers of a genetic change), recurrence risk is 25%. For cases resulting from an autosomal dominant genetic disorder that is inherited from a parent, recurrence risk can be up to 50%. For cases thought to result from a viral infection, the recurrence risk is likely to be very small.
References
- Norton ME, Chauhan SP, Dashe JS. Society for Maternal-Fetal Medicine (SMFM) clinical guideline #7: nonimmune hydrops fetalis. Am J Obstet Gynecol 2015; 212:127–139.
- Sparks TN, Thao K, Lianoglou BR, et al. Nonimmune hydrops fetalis: identifying the underlying etiology. Genet Med 2019; 21(6):1339-1344.
- Moreno CA, Kanazawa T, Barini R, et al. Non-immune hydrops fetalis: a prospective study of 53 cases. Am J Med Genet A 2012; 161: 3078–3086.
- Santo S, Mansour S, Thilaganathan B, et al. Prenatal diagnosis of non- immune hydrops fetalis: what do we tell the parents? Prenat Diagn 2011;31:186–195.
- Huang HR, Tsay PK, Chiang MC, et al. Prognostic factors and clinical features in liveborn neonates with hydrops fetalis. Am J Perinatol 2007; 24(1):33-38.
- Fukushima K, Morokuma S, Fujita Y, et al. Short-term and long-term outcomes of 214 cases of non-immune hydrops fetalis. Early Hum Dev 2011; 87:571–575.
- Mardy AM, Chetty SP, Norton ME, Sparks TN. A system-based approach to the genetic etiologies of non-immune hydrops fetalis. Prenat Diagn 2019; May 13. [Epub ahead of print]
- Kreger EM, Singer ST Witt RG, et al. Favorable outcomes after in utero transfusion in fetuses with alpha thalassemia major: a case series and review of the literature. Prenat Diagn 2016; 36(13):1242-1249.
*diagnostic information provided courtesy of our partners at UCSF, Department of Obstetrics, Gynecology, & Reproductive Sciences
Read more about Hydrops:
Fetal Hydrops: Successful In-Utero Surgery in the Pacific Northwest
Research Grant Awardee: Understanding the Underlying Causes of Hydrops